Novel 3D Imaging Method Provides JHU Scientists the Tool to Identify Metastatic Potential in Cancer Cells
Despite many landmark discoveries and developments in our 250-year-long efforts to fight against cancer, timely assessment of metastatic risk has proven to be one of the biggest challenges in the field. While molecular imaging of metastases has witnessed substantial progress, the metastatic phenotype of cancer cells remains an elusive realm that requires more strategic and practical approaches.
In 2020, a research team from Johns Hopkins University successfully captured even subtle morphological differences among three different cancer cell lines that vary in their metastatic competence. The results were published in the Journal of Biosensors and Bioelectronics[i]. In this study, Paidi[ii] et al. proposed a label-free route for optical phenotyping of live cancer cells at single-cell resolution.
This method claims to break away from many limitations in current clinical-standard assessments and common researching methods such as lymph node biopsies, genomic analyses of tumors, transcriptional analyses of breast cancer metastasis, or the use of epithelial markers in cell labeling, which are either invasive, high in false-negative rate, hard to prepare a sample or low in its sensitivity.
With an optical approach, Paidi’s research team employed 3D optical diffraction tomography (ODT) and label-free Raman spectroscopy to quantitatively investigate both morphological and molecular differences in three isogenic breast cancer cell lines.
Talking about HT-1, the holotomographic microscope produced by Tomocube company that was used for cell morphological assessment, Professor Barman [iii], a team member, acknowledged the system for allowing his team to tackle challenging research questions that they would not have been able to without it.
“The turn-key nature of the instrument coupled with its unique sensitivity and label-free attribute is a major boon for research laboratories that want to leverage the power of 3D quantitative phase imaging without having to expend significant resources and dedicate technical expertise in building such a system from scratch,” he explained.
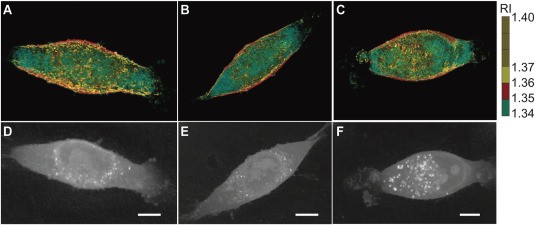

Holotomographic microscopy is a relatively new form of optical microscopy. It utilizes diffraction tomography to generate high-resolution, 3D holographic imaging of cells and their subcellular organelles. Vital information on unique cell properties such as cell volume, cell shape, cytoplasm density, cell dry mass, and cellular membrane dynamics can be quantified and generated for further analysis in a matter of seconds. Most importantly, cells will remain in their native state without being interfered with, damaged, or killed during the process of fixing and labeling.
After launching the HT-1 model in 2016, Tomocube Inc. continued to mark its legacy journey with the revolutionary HT-2 system in 2018. HT-2 is the world’s first microscope to combine 3D holograms and 3D fluorescence imaging in a single unit. There is no surprise that this disruptive technology was recognized as a photonics breakthrough by winning the Microscopy Today 2019 Innovation Award.
HT-2 incorporates a customizable 3-channel LED source (385 nm, 470 nm, 570 nm) and a motorized Z-drive (150 nm step resolution) to generate Z-stack images. A key aspect of the system is its correlative analysis in 2D, 3D, and 4D with both HT and fluorescence images performed by the company’s software – TomoStudio™.
While 3D refractive index (RI) tomograms are translated to cells’ morphological, chemical, and mechanical properties, the highly detailed fluorescence images provide more molecular specificity information. This advantage enables researchers to open new frontiers in bioscience without having to sacrifice the conventional research method they have been familiar with.
An increase in the adoption of holotomography in biology-related fields and growing awareness about the advantages of the technique in recent years suggest a prospect of a wide range of its potential applications. Details on current applications and publications which utilize HT series can be found Tomocube website page Applications and Publications.
i Santosh Kumar Paidi, Biosensors and Bioelectronics, https://doi.org/10.1016/j.bios.2020.112863
ii Dr. Santosh Paidi is currently a postdoctoral scholar in the School of Optometry at University of California, Berkeley. During his Ph.D. in Professor Ishan Barman’s lab at Johns Hopkins University, his research focused on the application of Raman spectroscopy and multivariate data analysis to develop novel quantitative approaches for addressing unmet needs in the molecular study of cancers.
iii Dr. Ishan Barman is an Associate Professor at the Department of Mechanical Engineering, with a joint appointment in the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center. His works focus on bioengineering, optics, and spectroscopy, aiming to develop integrative photonics solutions to complex problems in biological research and clinical diagnosis.