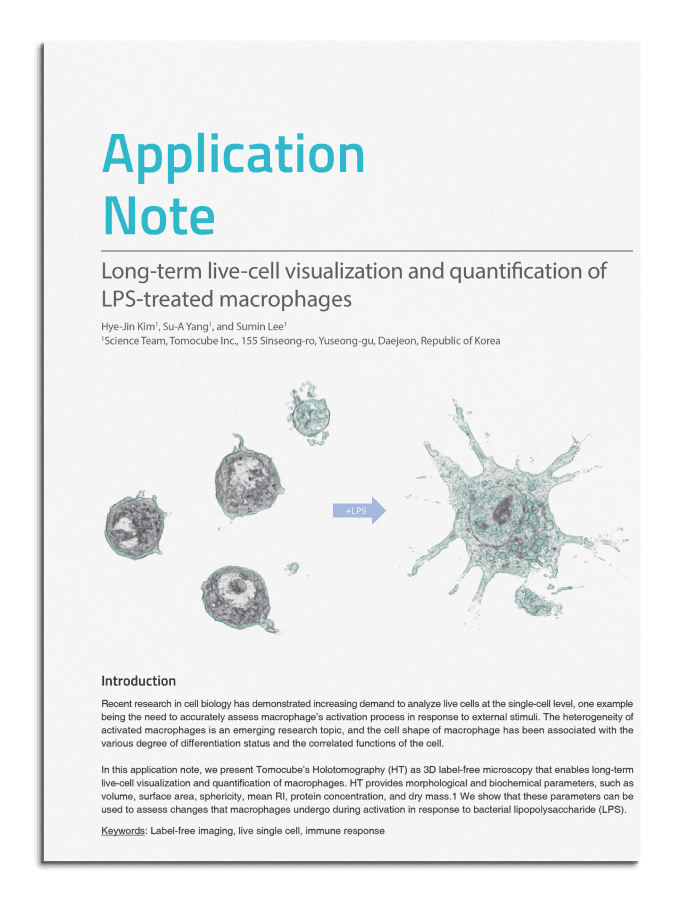
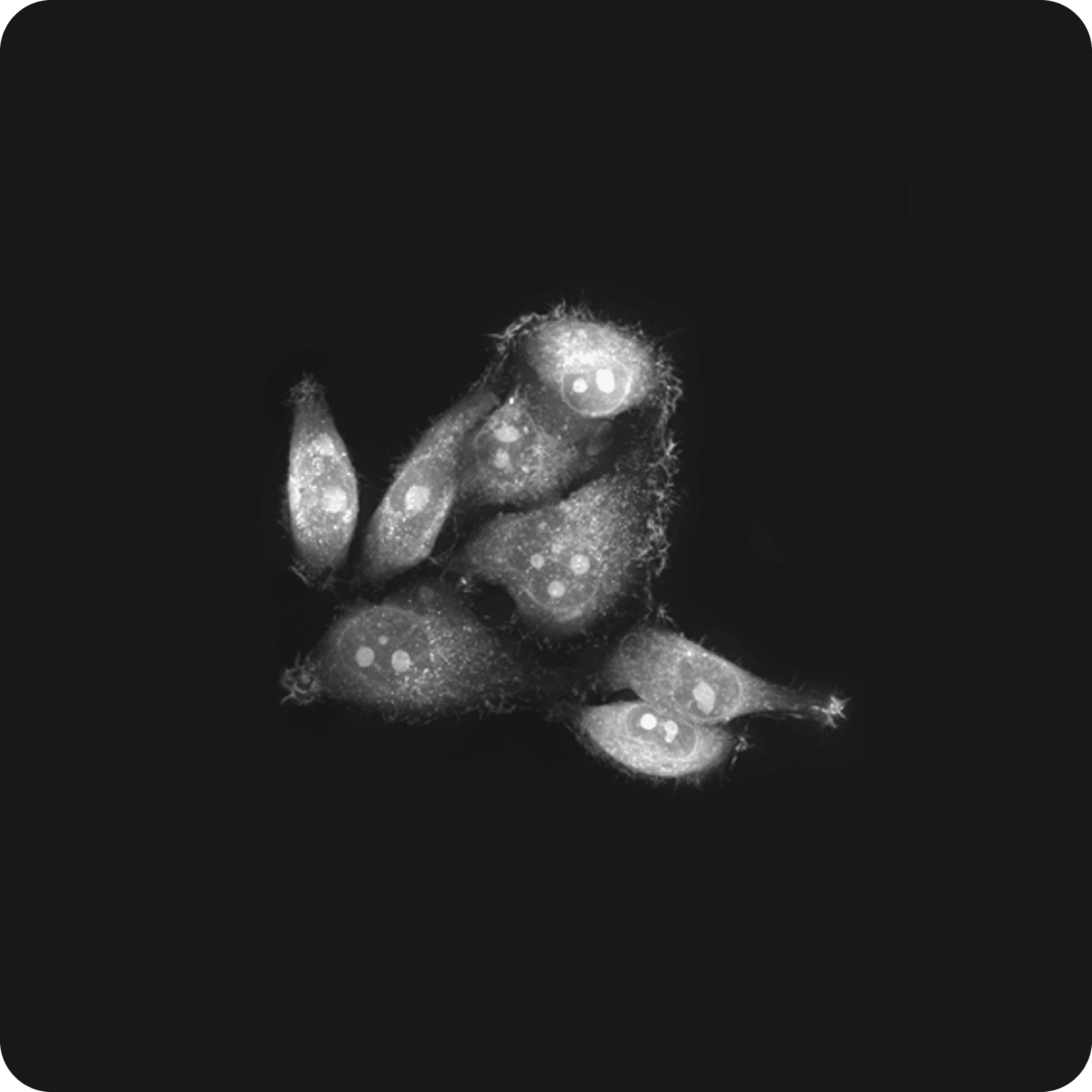Cell biology
Unlock the future of cellular research with Holotomography (HT), a cutting-edge imaging technology that transforms our understanding of cell biology. As the foundation of biological science, cell biology holds the key to unraveling the mysteries of life at the cellular level—essential for diagnosing diseases and pioneering new therapies.
Holotomography revolutionizes bio-imaging by offering a high-resolution, label-free, 3D, quantitative solution that leverages refractive index (RI) as an intrinsic contrast mechanism. This cutting-edge approach enables researchers to explore the intricate architecture of subcellular structures—like nuclei, mitochondria, lipid droplets— in fine detail, without relying on potentially harmful stains or labels during long-term observation, a requirement that is often unavoidable in other imaging techniques.
HT's innovative method safeguards the cells' natural physiology, eliminating the risks of phototoxicity and photobleaching. By maintaining the intact morphology of live specimens, HT can detect various microstructures without relying on fluorescence markers, which are often lost or degraded during staining and sample preparation processes. This ensures a more accurate and reliable analysis, preserving the true state of the cells under observation.
The benefits don’t stop there. HT offers full 3D visualization, giving researchers the ability to explore cellular structures from every angle, alongside quantitative measurements of cellular properties like dry mass and volume. This wealth of data provides deeper insights into cellular behavior and function, paving the way for breakthroughs in biomedical research.
Holotomography is more than just a tool—it's a revolution in cell biology, addressing the critical challenges of imaging with unparalleled accuracy and clarity. With HT, researchers are empowered to push the boundaries of discovery, unlocking new potential in the study of life at its most fundamental level.
Features
Discover Cell biology with HT
-
Live cell imaging
Live cell imaging is a crucial tool in cell biology, offering direct insights into how cells function in real time. HT enhances this capability significantly, allowing researchers to explore cellular complexities with exceptional clarity and precision.
As a label-free imaging technique, HT preserves the natural state and behavior of cells and their subcellular organelles. This non-invasive approach enables the study of various cellular processes over extended periods, including cell division, migration, cell interaction, signaling pathways, and cellular responses to drug-induced or environmentally induced changes.
The HT-X1 platform includes a stage-top incubator that ensures optimal culture conditions for long-term time-lapse imaging, maintaining cell health and viability throughout extended studies. This allows researchers to observe cellular processes over time without compromising the integrity of their observations. By reducing the need for time-consuming sample preparation and minimizing artifacts, HT streamlines the imaging process and enhances result accuracy.
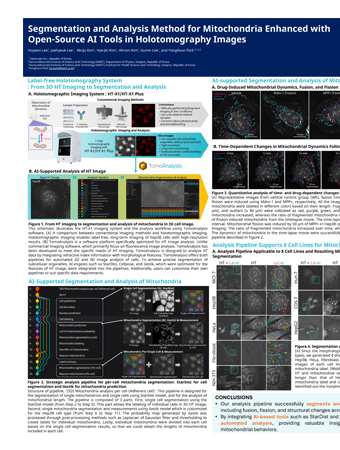
In the accompanying video, the response of Hep3B cells to elevated levels of reactive oxygen species (ROS) was monitored at 20-second intervals over 4.5 hours. A brief exposure of cancer cells to ROS, achieved by passing a 2.5 mM H2O2 solution through the ibidi µ-Slide, provoked phenomena such as cell swelling, mitochondrial fragmentation, cytoplasmic vacuolation, and cell death. -
Lipid droplet biogenesis
Lipid droplets (LDs) are essential subcellular organelles involved in lipid storage, energy metabolism, and various cellular processes. Despite their significance, the mechanisms underlying LD biogenesis and functions remain incompletely understood, necessitating advanced research tools for further investigation.
In LD research, HT offers a significant advantage by measuring the intrinsic RI value, which is higher for LDs compared to other cellular organelles, allowing for precise compartmentalization without the need for commonly used dyes like Nile Red or BODIPY. HT’s high-contrast, label-free imaging capabilities overcome the challenges of conventional methods, including low molecular specificity, photobleaching, and phototoxicity during prolonged imaging of live cells.
In a study published in ACS Nano, researchers analyzed the suppression of LD production over time in foam cells—key players in arteriosclerosis—when subjected to drug treatments (Park et al., 2020). The study involved examining various morphological and biophysical parameters, including the number, volume, and dry mass of LDs. Machine learning techniques were utilized to evaluate therapeutic effects at the single-cell level. With the ability to track individual LDs in three dimensions within living cells, HT shows potential for real-time therapeutic drug screening, including targeted nanodrugs, in lipid-containing cells.
Another study highlighted the potential of the HT-X1 platform by extensively observing a 42-day process wherein human knee chondrocytes underwent reverse differentiation to form dedifferentiated adipocytes (DFAT) and subsequently re-differentiated into mature adipocytes (Jeong et al., 2024). This application of HT allows for detailed and dynamic investigations into lipid droplet dynamics and related cellular processes. -
Cell-in-cell structures
Cell-in-cell (CIC) structures create a unique cellular arrangement where a whole cell is found in the cytoplasm of another. Unlike apoptotic cells that are engulfed through phagocytosis, the internalized cells in CIC structures remain viable for extended periods and often exhibit dynamic activities before determining their fate. Considerable evidence suggests that CIC structures play a significant role in both pathological and physiological conditions, with their most frequent occurrence being in cancer tissues.
HT is particularly well-suited for research elucidating the mechanisms and consequences of CIC formation. The capacity of HT to monitor the long-term cellular dynamics in real time, without the need for multiple cell-specific markers, makes it an invaluable tool for investigate diverse cell interactions in their native state.
In a recent study published in iScience, the CIC phenomenon, where NK cells entered cancer cells, was identified using HT imaging (Choe et al., 2022). Utilizing label-free observation of this CIC structure, the study focused on heterotypic cell segmentation, facilitating a further analysis of its contribution to drug resistance. Continuous monitoring of the time-lapse process of cancer cell death added temporal insights. Incorporating correlative fluorescence imaging aided in distinguishing different cell types, enhancing the understanding of dynamics within cell-in-cell structures.
Results show that CIC-forming cancer cells showed lower response to NK cytotoxicity, higher proliferative ability, and higher resistance to anticancer drugs than non-CIC cancer cells. It suggests the use of heterotypic CICs as a functional biomarker to predict NK susceptibility and drug resistance in solid cancer cell lines. -
Cell migration
Cell migration is vital for biological processes such as embryonic development, wound healing, and immune responses, and it also play a significant role in pathological conditions like cardiovascular diseases and cancer metastasis. Understanding cell migration in both 2D surfaces and complex 3D matrices can offer insights into these conditions and improve treatments.
Live cell timelapse imaging is commonly used in migration assays to explore dynamic processes like wound healing and cancer cell metastasis. HT is a key tool in this research, allowing real-time, non-invasive tracking of cell migration.
In wound healing, for example, fibroblasts and endothelial cells migrate to injury sites to promote tissue repair. The accompanying video showcases wound healing assay, a standard in vitro technique for probing collective cell migration. An artificial cell-free area was created in a confluent monolayer of NIH-3T3 embryonic fibroblast. Through tile and timelapse imaging at 10-min intervals over 36 hours, HT captured the entire closure process in high resolution for detailed analysis.
Another case study features migrasomes—extracellular vesicles formed in migrating cells and are gaining renewed attention in cellular biology. In a recent study, HT imaging was employed to observe that TNF alpha increased the formation of migrasomes (Gagat et al. 2021). HT played a crucial role in correlating the 3D morphology with localization in correlative fluorescence imaging, enabling stereoscopic observation of migrasomes.
Resources
Selected publications
-
Researchers utilized HT for label-free, 3D, timelapse monitoring of living foam cells to assess the therapeutic impact of designed nanodrugs on lipid droplet production in macrophages and foam cells - a crucial feature in arteriosclerosis. Machine-learning-based image analysis was also employed for single-cell level therapeutic evaluation.
-
HT was used to assess the uptake of hyaluronic acid-based nanoparticles (FHA-NPs) and its effect on cell morphology and organelle dynamics in lung carcinoma epithelial cells. 3D refractive index images helped confirm the important role of lipid metabolism in ferroptosis through the evident accumulation of lipid droplets/cytoplasmic lysosomes in treated cells.